14.7. Flamecooler#
| Author: | Kirsten Stadermann |
| Time: | 5-10 minutes |
| Age group: | from grade 8, extension available for grade 11 and above |
Introduction#
A burning candle is an everyday phenomenon, but the physical and chemical effects that contribute to the burning are complex. This demonstration is suitable for developing your students’ physical reasoning. Without complicated equipment, you show a surprising phenomenon. Why does the flame of a candle disappear?
Materials Needed#
Approximately 20 cm long, thick (Ø 1 to 2 mm) uninsulated copper wire
Candle
Lighter or matches
Preparation#
Make a 1-2 cm long spiral from the copper wire by wrapping it around a pencil. The coils should not be too tight together, otherwise, you won’t see the flame, but if the coils are too far apart, the effect is lessened. Test in advance!
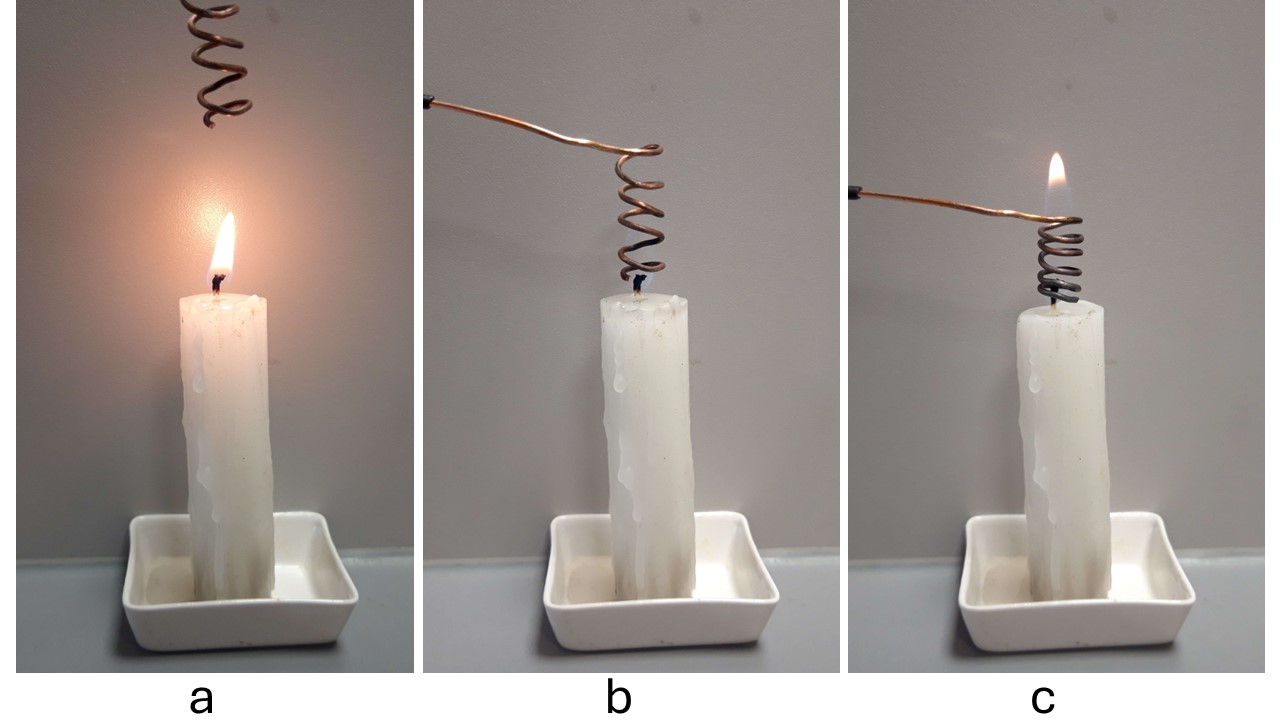
Fig. 14.14 The visible flame of a candle disappears near a copper spiral.#
Procedure#
Depending on the candle and the spiral, the experiment will be slightly different for everyone. Proper observation is therefore important.
Light the candle.
Slowly bring the spiral from above over the candle flame. Do not touch the wick with the spiral.
Write down what you observe as precisely as possible. What surprises you?
The visible flame will disappear at the spiral. If the spiral is loosely wound, you will still see a thin yellow flame in the center and a blue one around it. In other cases, the flame will go out. If smoke still rises, you can relight the flame above the spiral.
Alone or in pairs, explain what you see. Write down your hypothesis.
Collect the answers and ask for a substantiation of their hypotheses. This way, students learn to make connections between physical concepts and their own experiences. Do not reject any explanation.
A question to check students’ understanding: Think of an experiment to test your hypothesis.
Physics background#
For age 10-12:#
Briefly: three things are needed for burning: fuel, oxygen, and a sufficiently high temperature. If you remove one of these things, the combustion stops. Here, you lower the temperature.
More extensively: To keep a candle burning, the wax must be in a gaseous state. You can show that solid wax does not burn. To burn gaseous wax with oxygen, a certain temperature is needed. Copper is a much better heat conductor than air. The copper spiral ensures that the temperature of the gaseous wax drops below that ignition temperature. The flame goes out. The gaseous wax still rises through the spiral, so there is enough fuel. You can relight this fuel above the spiral.
For age 16-18:#
For combustion, activation energy (heat) is needed. A burning candle provides this energy itself since it is an exothermic process. If you now hold the copper spiral in the flame, the released energy is dissipated so that the heat is no longer available as activation energy. The flame goes out.
That the flame in the coil can turn blue is surprising to many because we know from the gas burner that the blue or transparent flame (complete combustion) is hotter than the yellow flame (incomplete combustion). With a candle, it is more complicated. The natural gas from the gas burner can react directly with oxygen in the air. With a candle, the liquid wax must first evaporate, which requires a lot of energy, leading to a relatively low temperature (600°C). Subsequently, long chains of wax vapor (e.g., palmitic acid \(\text{CH}_3[\text{CH}_2]_{14}\text{COO}[\text{CH}_2]_{29}\text{CH}_3\)) are thermally broken down, which again costs energy (temperature in this area: 800-1000°C). Only then does an exothermic reaction with oxygen (oxidation) occur. The temperature is around 1200°C. The copper spiral removes heat and creates an area where the molecular chains still need to be broken down over a longer distance.
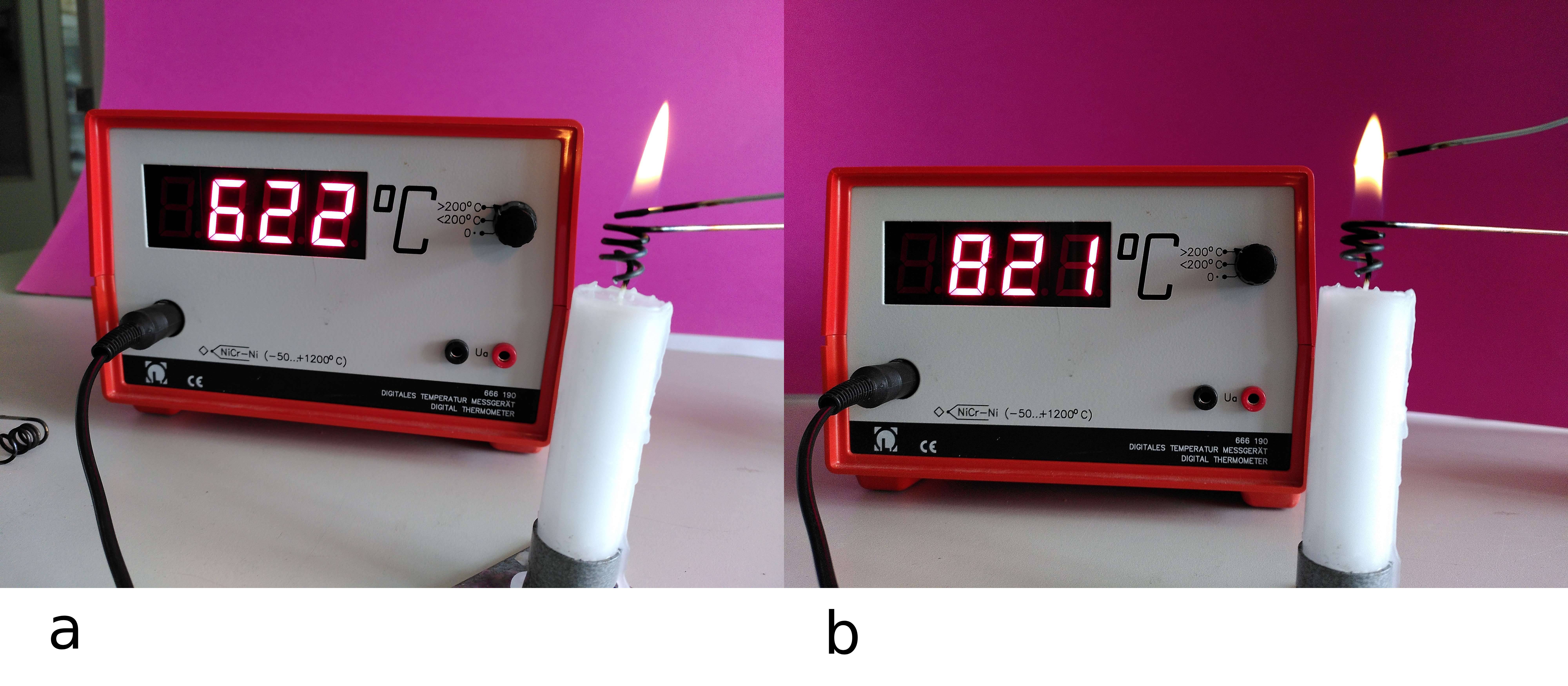
Fig. 14.15 A measurement of the temperature in (a) the invisible part and (b) the visible part of the flame.#
In Figure 14.15, you see an attempt to measure the temperature by holding a thermocouple in different parts of the flame: The actual temperature of the flame will be higher than the indicated value because the temperature sensor itself also conducts heat and therefore causes cooling. It is clear that the yellow part of the flame is hotter than the transparent part.
