14.1. Can it be air?#
Reasoning about flames and vapor
| Author: | Peter Dekkers |
| Time: | 5-10 minutes |
| Age group: | 12 - 14 |
| Concepts: | Phases of water, evaporation, condensation, combustion, air, oxygen |
Introduction#
Just above the bubbling surface of boiling water, nothing unusual seems to be happening. Slightly higher, however, a kind of fog is visible, and rising. An object placed in that fog gets wet: the fog consists of water droplets. But then, what fills the space between the bubbling water and the fog? Is that hot air, or not? How could we find out?
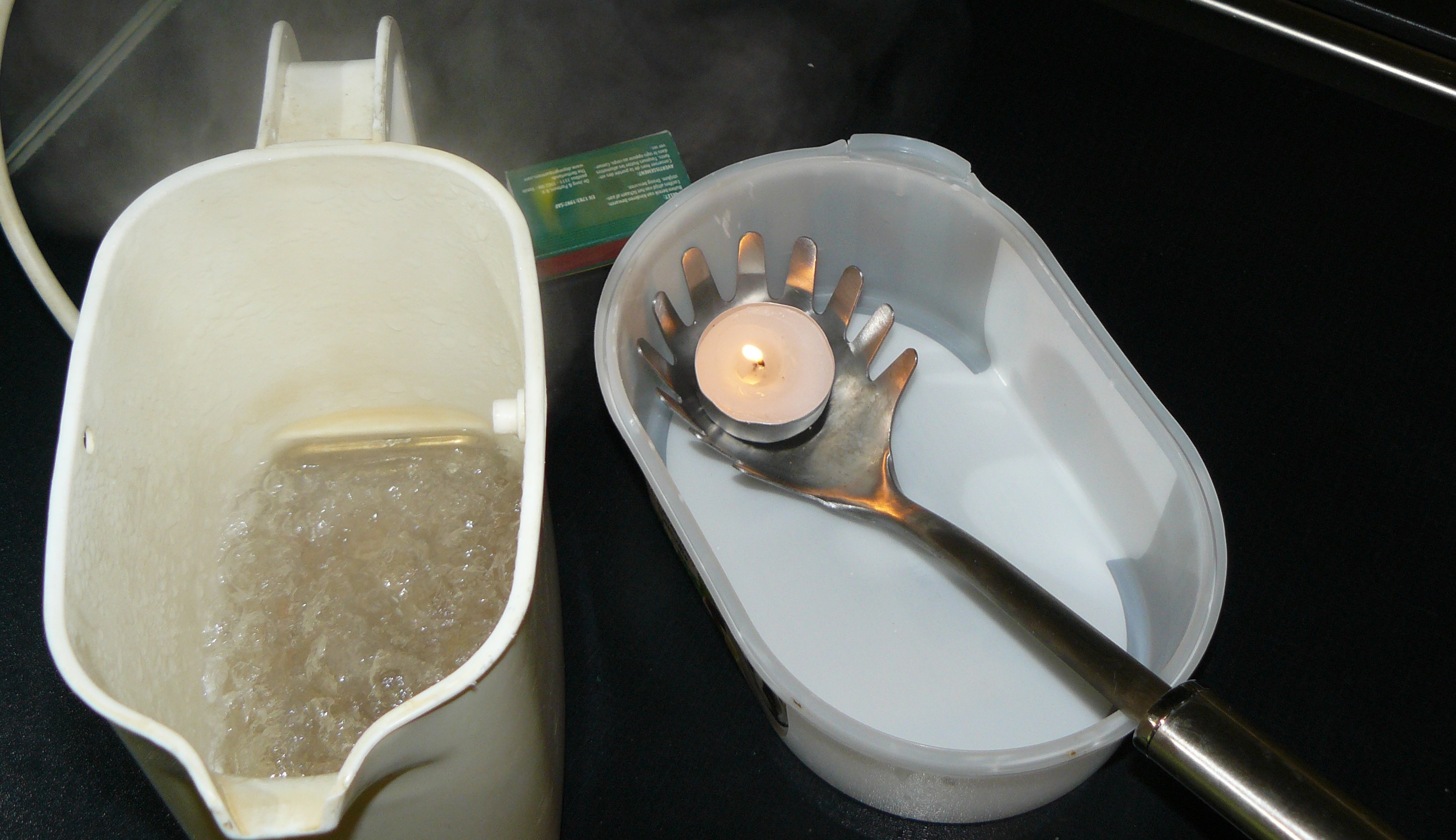
Fig. 14.1 The required materials#
Equipment#
Electric boiler
Water
Tealight
A spaghetti spoon
Matches
Webcam and screen to make the candle in the spoon visible to the whole class
Preparation#
Pour some water in the kettle and let it come to a boil.
Leave the lid open so that the kettle does not automatically switch off.
Place the tealight in the spoon and light it.
Warning
Working with boiling water has risks. Do not let students come too close; if possible, use a camera and projector to give everyone a good view.
Procedure#
Discuss the questions in the introduction with the students. Between the bubbling liquid in the kettle and the rising fog above it, you see ‘nothing’. What is actually there? Most students will probably say ‘(hot) air’. That idea can be tested, because if it is ‘just’ air, like the air we breathe, there must be oxygen in it. So if we hold a burning candle in the kettle, it would then just keep on burning.
Bring the burning candle down into the kettle, and note that the flame dies. Summarize the observations:
The flame dies: above the water is a substance that looks like air but apparently is not.
Bubbles come out of the water.
Water droplets float above the kettle.

Fig. 14.2 left: Above the kettle, the flame burns somewhat weaker.
right: Inside the kettle, above the water, the flame extinguishes#
Draw a conclusion together. It may well be that the conclusion is not: there is water vapor above the liquid. After all, that has not been proven. What we can agree on is:
What comes out of the bubbles and hangs above the liquid looks like air.
It is not air, because a candle does not keep burning in it.
This substance turns into water droplets when it rises higher.
The substance concerned has a name: steam. Often the fog is said to be made of steam, but that is not scientifically correct. You could conclude the demonstration together through establishing a proper name for the droplets hanging above the kettle. ‘Cloud’, ‘mist’ and ‘fog’ are acceptable names, but your students may think of more imaginative ones.
Physics background#
The air that is above the liquid before the water boils is quickly pushed away when the liquid starts to boil. The temperature inside the kettle remains at 100°C. Water vapor or steam fills the space immediately above the boiling surface, it condenses only a bit higher up above the kettle, where the temperature is lower. Due to lack of oxygen, the flame dies in the steam (some prior knowledge about burning is prerequisite).
