20.5. Boiling without heating in a vacuum#
| Author: | Paul Hupkens and Ed van den Berg |
| Time: | 15 minutes |
| Age group: | Grade 8-12 |
| Concepts: | Air pressure, pressure-volume relationship, air pressure-boiling point relationship |
Introduction#
Expanding shaving cream or balloons, boiling water without heat supply, and more possibilities with the vacuum bell jar.
Equipment#
Bell jar with vacuum pump
Balloon
Sponge
Shaving cream or marshmallows
100 mL graduated cylinder
Beaker with room temperature water
Thermometer (non-mercury)
Safety goggles
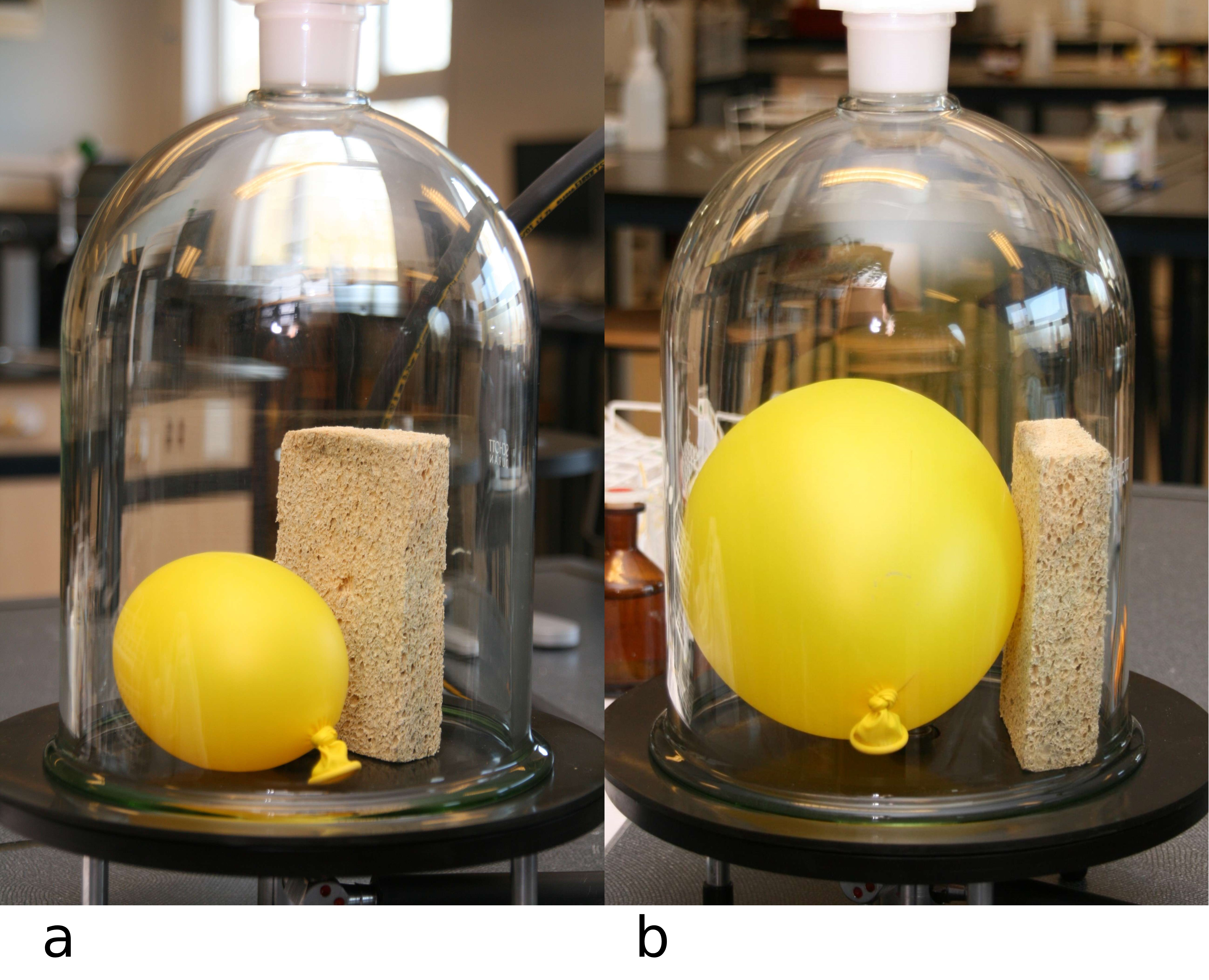
Fig. 20.14 Inflating a balloon and a sponge under the bell jar.#
Preparation#
Set up all materials. Pre-inflate the balloon a little and tie it off.
Procedure#
I have a cylinder here and will put some shaving cream in it. Next, we will start vacuuming out the air. What will happen to the shaving cream? Write down your prediction and explain your reasoning (predict-explain).
Perform the demonstration: the shaving cream swells up. Explain why! (observe-explain). Air is trapped in closed pockets within the shaving cream. As the air pressure outside the cream decreases, these pockets of air expand until the air pressure inside and outside the pockets is equal.
Fig. 20.15 Shaving cream.#
Next, place a glass of water with a thermometer under the bell jar. What will we see and why? (predict-explain). Students may not have any idea. In that case, just proceed with the demonstration.
The water starts to boil (observe) without any added heat. Bubbles form at the bottom of the glass and rise to the top. How is this possible? What is boiling? What does pressure have to do with it?
Fig. 20.16 Boiling water at room temperature under the bell jar#
What happens to the temperature during the ‘boiling’? Why do you think that is? Students can predict here.
The temperature should drop because the phase transition requires energy (the water may actually freeze if only a little bit of water is used!).
Finally, place a slightly inflated balloon and a sponge under the bell jar, see Figure 20.14, and ask students to predict what will happen to both. Will the balloon get bigger, smaller, or stay the same? Why? Will the sponge get bigger, smaller, or stay the same? Why? Perhaps place a wager.
Predictions might be well-reasoned, such as the sponge swelling because air pockets inside it expand. This turns out not to happen—the conclusion being that the air within the sponge can freely move out.
Physics background#
With shaving cream, we are dealing with trapped air pockets that expand when the pressure outside the shaving cream decreases.
With water, we revisit what boiling is exactly. Normally, the air pressure in bubbles of evaporated water is equal to the outside air pressure. This happens at 1 atmosphere of external pressure, so at 1 atmosphere, the pressure inside the bubbles is less and they collapse at lower temperatures. However, if the external pressure is much lower, as in the bell jar, bubbles of evaporated water can survive at much lower temperatures, and thus the water boils at a much lower temperature. This can also be shown without a vacuum bell jar, for example, with warm water (starting at around 60 °C) in a wine bottle and a wine pump. It can also be done by heating a flask of water to boiling, stopping the heating, and sealing it with a cork/stopper. Inverting the flask and pouring cold water over the raised bottom of the flask makes the water boil again due to the low pressure created when water vapor condenses (Liem [1987] p. 106). Lastly, the sponge neither gets bigger nor smaller. Apparently, there are good connections between the gaps in the sponge, allowing air to easily move in and out.
Tip
When boiling under the bell jar: once the water boils, turn off the pump to prevent much condensation inside.
Demonstrating boiling at a lower temperature can also be done by boiling water in a flask, immediately turning off the heat, sealing the flask with a rubber stopper and thermometer in the stopper, and then inverting it and pouring cold water over it. The reduced pressure brings the water to a boil again at a temperature below 100 degrees.
References#
Tik L Liem. Invitations to science inquiry 2nd edition. ERIC, 1987.
